Organic chemistry
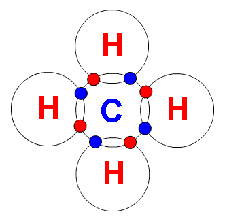
Aliphatic hydrocarbons
1. Alkanes (Saturated Hydrocarbons)
- Structural Isomerism
- IUPAC (International Union of Pure and Applied Chemistry) nomenclature of alkanes
- Properties of Alkanes
- Reactions of Alkanes
- Alkyl Halides
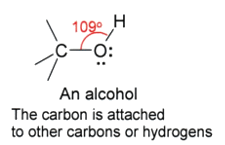
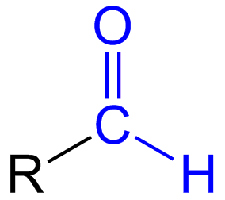
The Carbonyl Group
Aldehydes and ketones both contain the carbonyl group.
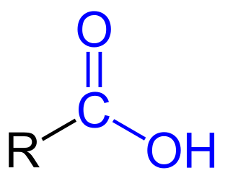
Carboxylic Acids
A carboxylic acid is an organic compound that contains a carboxyl group, –COOH.
Carboxyl group - a carbon atom that is double-bonded to 1 oxygen atom and single-bonded to a hydroxyl group.
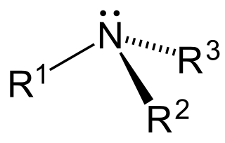
Types of amines – primary, secondary, tertiary, quarternary amines
An amine is a derivative of ammonia (NH3) in which one or more of the hydrogen atoms are replaced with a substituent groups such as an alkyl or aryl groups.
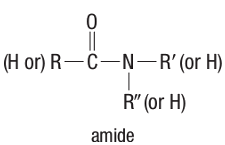
Amide structure
Amide - an organic compound that contains a carbonyl group (-CO-) bonded to a nitrogen atom.
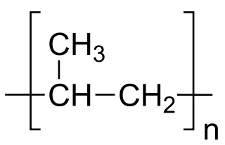
A polymer is a very large molecule that is built from monomers (the repeating units that make up a polymer).
Many different biomolecules, such as DNA, proteins, and carbohydrates, are polymers.
