Basic concepts of thermochemistry
Thermochemistry - the study of the energy changes that accompany physical or chemical changes in matter.
Work - the amount of energy transferred by a force over a distance; SI units - joules (J).
Energy - the ability to do work; SI units - joules (J).
| Energy | |
| Potential energy the energy of a body or system due to its position or composition |
Kinetic energy the energy of an object due to its motion |
Thermal energy - the total quantity of kinetic and potential energy in a substance.
Heat - the transfer of thermal energy from a warm object to a cooler object.
Temperature - a measure of the average kinetic energy of entities in a substance.
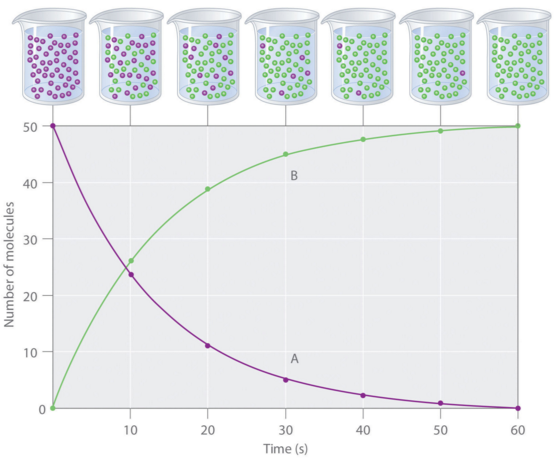
Chemical kinetics - the area of chemistry that deals with rates of reactions.
Reaction rate - the change in concentration of a reactant or a product of a chemical reaction per unit time.
Commonly used methods include tracking changes in gas volume, colour, mass, pH, and electrical conductivity.