| Strong Acid/Base | Weak Acid/Base |
|
Strong acid - ionise/dissociate completely producing H+ ion Strong base - ionise/dissociate completely producing OH- ion All are in ions state, No molecule left Strong electrolyte with high conductivity |
Weak acid - ionise/dissociate partially producing H+ ion Weak base - ionise/dissociate partially producing OH- ion Most in undissociated molecule form Poor electrolyte with low conductivity |
| HCl → H+ + Cl- | CH3COOH ⇌ H+ + CH3COO- |
Stronger acids and bases have ionization equilibrium positions farther to the right.
Weaker acids and bases have equilibrium positions farther to the left.
Thus, in water, strong acids or bases will ionize more than weak acids or bases.
Contents:
Strong Acids and Weak Acids
Polyprotic Acids
Oxyacids and Organic Acids
Percent Dissociation
Strong Bases and Weak Bases
Strong Bases
Weak Bases
Water as an Acid and a Base
The Relationship between Kw, Ka, and Kb
pH and pOH
Strong and Weak Acids
When acids dissolve and ionize in water, they form a dynamic equilibrium between reactants and products.
Strong acid - an acid that ionizes almost 100% in water, producing hydrogen ions.
The concentration of hydronium ions (H3O+) in a dilute solution of a strong acid is equal to the concentration of the acid.
Strong acids - hydrochloric acid, HCl; hydrobromic acid, HBr; hydroiodic acid, HI; nitric acid, HNO3.
Weak acid - an acid that only partly ionizes in water, producing hydrogen ions.
Weak Acids - Tylenol (acetaminophen, Ka - 1.2 x 10-10) and Aspirin (acetylsalicylic acid, or ASA, Ka - 3.27 x 10-4).
Strong and weak acids

(a) A strong acid is almost completely ionized in water, resulting in relatively high concentrations of H+ (aq) and A- (aq) ions and a much lower concentration of HA (aq) molecules.
(b) A weak acid consists of a relatively high concentration of non-ionized HA(aq) molecules and much lower concentrations of H+ (aq) and A- (aq) ions.
Acid Strength
| Property | Strong acid | Weak acid |
| Value of acid ionization constant, Ka | Ka is large | Ka is small |
| Position of the ionization equilibrium | far to the right | far to the left |
| Equilibrium concentration of H+ (aq) compared with original concentration of HA | [H+ (aq)]equilibrium ~ [HA (aq)]initial | [H+ (aq)]equilibrium << [HA (aq)]initial |
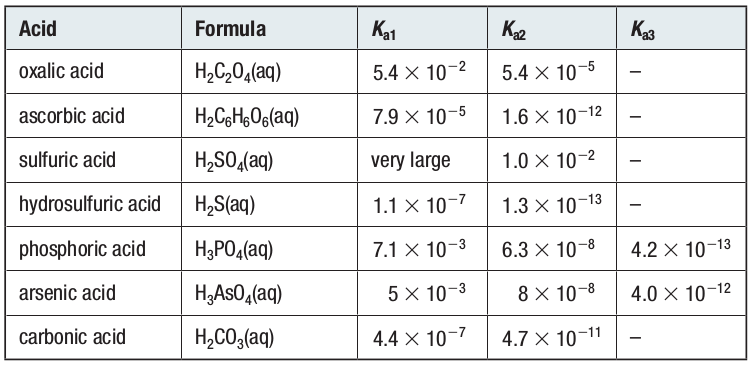
Values of Ka for Some Common Acids

The relationship of acid strength and conjugate base strength
HA (aq) + H2O (l) ⇌ H3O+ (aq) + A- (aq)
The relationship of acid strength and conjugate base strength

The stronger an acid, the weaker its conjugate base, and conversely, the weaker an acid, the stronger its conjugate base.
Polyprotic Acids
A few acids contain only a single hydrogen ion that can dissociate. These acids are called monoprotic acids.
Strong monoprotic acids - hydrochloric acid, hydrobromic acid, hydroiodic acid.
Weak monoprotic acid - hydrofluoric acid, HF.
Many acids contain two or more hydrogen ions that can dissociate.
Acids that contain two hydrogen ions dissociate to form two anions are sometimes called diprotic acids.
The acid that is formed by the first dissociation is stronger than the acid that is formed by the second dissociation.
Sulfuric acid, H2SO4 (aq), has two hydrogen ions that can dissociate.
Sulfuric acid is a strong acid. This is true only for its first dissociation, however.
H2SO4 (aq) → H+ (aq) + H1SO4- (aq)
The resulting aqueous hydrogen sulfate ion, HSO4-, is a weak acid.
It dissociates to form the sulfate ion in the following reversible reaction:
HSO4- (aq) ⇌ H+ (aq) + SO42- (aq)
Acids that contain three hydrogen ions are called triprotic acids. Phosphoric acid, H3PO4 (aq), is a triprotic acid. It gives rise to three anions.
All polyprotic acids, except sulfuric acid, are weak.
Their second dissociation is much weaker than their first dissociation.
For this reason, when calculating [H3O+] and pH of a polyprotic acid, only the first dissociation needs to be considered.
Acid Ionization Constants for Polyprotic Acids at SATP
Oxyacids and Organic Acids
Oxyacid - an acid in which the acidic hydrogen atom is attached to an oxygen atom.
| Strong oxyacids | Sulfuric acid, H2SO4 |
| Weak oxyacids | Phosphoric acid, H3PO4 (aq); nitrous acid, HNO2 (aq); hypochlorous acid, HClO(aq) |
Organic acid (carboxylic acid) - an acid (except carbonic acid, H2CO3 (aq)) containing carbon, oxygen, and hydrogen atoms.
An organic acid has a carbon backbone and a carboxyl group (-COOH).
Most organic acids are weak acids.
Percent Dissociation
The percent dissociation of a weak acid is the fraction of acid molecules that dissociate compared with the initial concentration of the acid, expressed as a percent.
The percent dissociation depends on the value of acid ionization constant (Ka) for the acid, as well as the initial concentration of the weak acid.

Strong Bases and Weak Bases
Strong Bases
Strong base - a base that dissociates completely in water, producing hydroxide ions.
All the hydroxides of the Group 1 (LiOH, NaOH, KOH, RbOH, CsOH) and the Group 2 (alkaline earth - Ca(OH)2, Ba(OH)2, Sr(OH)2) elements are strong bases. Although the alkaline earth hydroxides are strong bases, they are only slightly soluble.
Weak Bases
Weak base - a base that only partially reacts with water to produce hydroxide ions.
Many compounds are bases even though they do not contain the hydroxide ion. These compounds increase the concentration of hydroxide ions in aqueous solution because of their reaction with water. These bases are Brønsted–Lowry bases.
NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH- (aq)
In this reaction, water is a Brønsted–Lowry acid and ammonia is a Brønsted–Lowry base.
Since the equilibrium position of this reaction is far to the left, ammonia is considered to be a weak base. Bases such as ammonia have at least one unshared pair of electrons that is capable of forming a coordinate covalent bond with a hydrogen ion.
Water as an Acid and a Base
Water is the most common amphiprotic substance: it can behave as either an acid or a base.
Water can behave as both an acid and a base in the same reaction. This reaction is called the autoionization of water.
Autoionization of water - the transfer of a hydrogen ion from one water molecule to another.
A chemical equation for the autoionization of water:
2H2O (l) ⇌ H3O+ (aq) + OH- (aq)
An equilibrium law equation for this reaction:
K = [H3O+ (aq)][OH- (aq)] / [H2O (l)]2
Simplified equation:
Kw = [H+ (aq)][OH- (aq)]
Ion-product constant for water (Kw) - the equilibrium constant for the autoionization of water.
At 25 °C in pure water:
[H+ (aq)] = 1.0 x 10-7 mol/L
[OH- (aq)] = 1.0 x 10-7 mol/L
The value of Ion-product constant for water (Kw) at 25 °C:
Kw = [H+ (aq)][OH- (aq)] = (1.0 x 10-7)(1.0 x 10-7) = 1.0 x 10-14
In any aqueous solution at 25 °C, no matter what the solution contains, the product of [H+ (aq)] and [OH- (aq)] must always equal 1.0 x 10-14.
| A neutral solution | [H+ (aq)] = [OH- (aq)] |
| An acidic solution | [H+ (aq)] > [OH- (aq)] |
| A basic solution | [OH- (aq)] > [H+ (aq)] |
The Relationship between Kw, Ka, and Kb
There is an important relationship between the dissociation constant for an acid, Ka, and the dissociation constant for its conjugate base, Kb.
The ionization reaction of a weak acid, HA(aq), is represented as:
HA (aq) + H2O (l) ⇌ H3O+ (aq) + A- (aq)
The acid ionization constant equation is :
Ka = [H3O+ (aq)][A- (aq)] / [HA (aq)]
A-, the conjugate base of HA, is itself a base. An ionization equation for the reaction of A- with water:
A- (aq) + H2O (l) ⇌ HA (aq) + OH- (aq)
The corresponding base ionization constant equation:
Kb = [HA][OH-] / [A-]
The product KaKb gives an interesting result.
KaKb = [H3O+ (aq)][A- (aq)] / [HA (aq)] x [HA][OH-] / [A-] = [H3O+ (aq)][OH-] = Kw
KaKb = Kw
One interpretation of the result is the stronger an acid, the weaker its conjugate base must be.
This makes sense chemically, because a strong acid gives up a proton from each molecule. Therefore, its conjugate base does not bond with the proton.
In summary, then, the strength of an acid and its conjugate base are inversely related.
The conjugate of a strong acid is always a weak base, and, conversely, the conjugate of a strong base is always a weak acid.
The relative strengths of conjugate acid-base pairs

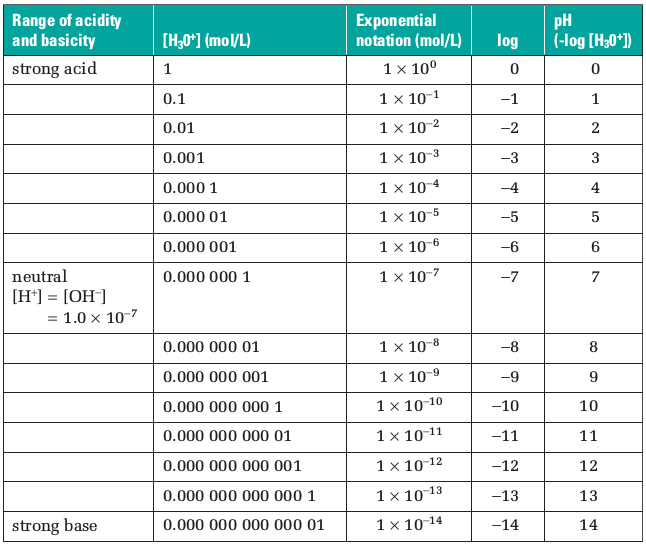
pH and pOH
The negative logarithm of the hydrogen ion concentration is called pH.
The negative logarithm of the hydroxide ion concentration is called pOH.
pH = -log[H+ (aq)]
pOH = -log[OH- (aq)]
Since pH is a logarithmic value based on 10, the pH changes by 1 for every 10-fold change in [H+ (aq)].
And since pH is defined as –log[H+ (aq)], pH decreases as [H+ (aq)] increases and vice versa.
The pH of common aqueous solutions at 25 °C ranges from 0 to 14.
In pure water pH = 7 and pOH = 7.
Understanding pH